Orange County Research Center:
Where cutting-edge clinical trials meet compassionate care
We conduct all of our drug and medical device trials with the highest standards of precision, safety, and care
Who we are
Advancing medical science, one clinical trial at a time
- An experienced team – We have completed over 2,000 studies, with our seasoned researchers and medical professionals upholding the highest quality standards.
- Diverse patient population – We have access to a diverse patient pool, allowing us to recruit targeted populations to meet specific trial needs.
- A state-of-the-art facility – Our facility for Phase I–IV trials is equipped with advanced technology and comfortable patient accommodations.
- Rapid turnaround times – Our efficient processes and streamlined operations enable us to quickly conduct studies.
Phase 1 Unit
Phase I Unit: Setting the standard for early-phase trials
- 50-bed facility with 16 telemetry beds
- Dedicated rooms for special procedures
- Catered meals with dietary accommodations
- Comfortable environment for participants

Equipment
State-of-the-art on-site trial equipment for precision research
Diagnostic and monitoring tools
- Telemetry
- Treadmill
- Fibroscan
- DEXA scan
- ECG machines
- Ultrasound
- Echocardiography
- Six-minute walk testing
- 24-hour ambulatory blood pressure monitoring (15 machines)
Laboratory and support equipment
- Freezers and refrigerators (five total, including −80°C)
- Continuous temperature monitoring tools
- Refrigerated and ambient centrifuges
- Strength testing devices (hand grip)
- Infusion pumps
- Weight scales
In-house procedures
Comprehensive in-house procedures for reliable data
Fibroscan
Assesses liver fibrosis and steatosis
Ultrasound
Reviews abdominal, cardiac, and vascular structures and functions
Echocardiography
Evaluates heart structure and functions
Radial arterial lines
Provide continuous monitoring and accurate blood sampling for real-time physiological assessments
Treadmill stress testing
Assesses cardiovascular health and exercise capacity
Skin biopsy
Collects skin samples for dermatological evaluations and research
Muscle biopsy
Analyzes muscle tissue for diagnostic and research purposes
DEXA scanning
Measures bone density and body composition to assess risks of osteoporosis and other conditions
Unique and complicated protocols
Expertise in complex protocols
- Long-term bed rest studies
- Thorough QT studies
- Holter monitoring studies
- ABPM studies
- NASH and MASH studies requiring liver biopsies
- Studies requiring muscle biopsies
- Studies requiring skin biopsies
- Studies requiring placement of radial arterial lines
- Studies requiring cold pressor testing
- Food effect studies
- In the process of developing the capabilities of performing euglycemic insulin clamp studies with in-house endocrinologist

Testing protocols
Specialized testing protocols for every study
- Renal dysfunction studies
- Hepatic dysfunction studies
- IV infusion studies
- Subcutaneous and IM injection studies
- Vaccine studies
- Topical studies
- Nasal administration studies
- Optometric studies
- Long-term bed rest studies
QT studies
Navigate the regulatory landscape with OCRC’s TQT expertise
Proven track record
Over the past six years, OCRC has successfully conducted 10 TQT studies.
Comprehensive capabilities
Our team is experienced in studies requiring telemetry, Holter monitoring, and multiple ECG recording.
Device studies
Advancing the future of medical devices
- Multiple innovative wrist and finger continuous monitoring BP devices and comparing them to intra-arterial measurements for FDA submissions
- Noninvasive glucose measuring devices
- Measurement of arterial compliance
- New insulin pump devices
- Continuous glucose monitoring devices
- Gastric PH capsule

Diversity in participants and staff
A diverse approach to clinical trials for inclusive results
The OCRC database reflects this diversity, with an approximate ethnic breakdown of:
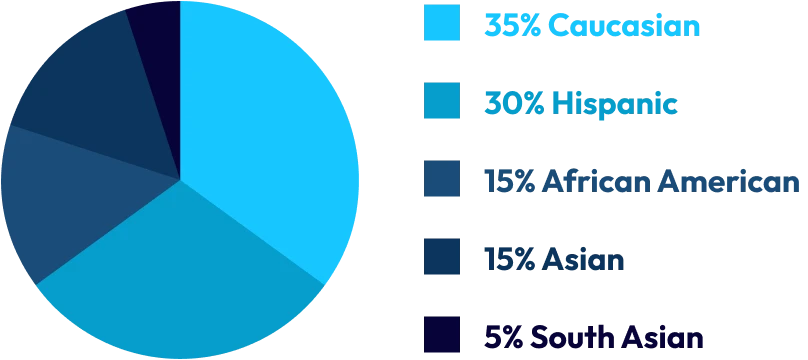
To support this diversity, our team is equally multicultural and multilingual, with staff fluent in:
- Spanish
- Mandarin
- Cantonese
- Japanese
- Tagalog
- Hindi
- Vietnamese
Protocol review
Expert protocol review and guidance for seamless trials
Review and comment on study protocols
Review study Informed Consents
Review comprehensive study reports
Serve as a Lead Investigator for the study program
At the sponsor’s request, present data from studies at medical conferences
Added value
Orange County Research Center’s added value to studies
Protocol review expertise
We thoroughly review study protocols to optimize workflow within the clinical environment while ensuring the collection of all required data.
Informed Consent form (ICF) development
Our team reviews and creates clear, comprehensive ICFs, ensuring participants fully understand the study and their rights.
Source document creation
We develop detailed, customized source documents to facilitate accurate and consistent data capture.
Active participation in safety oversight
We play a vital role in DLRM safety meetings, providing valuable input to ensure participant safety throughout the study.
Lead investigator role
OCRC frequently serves as the lead investigator, coordinating efforts across multiple study sites to ensure consistency and adherence to study protocols.
Comprehensive study report (CSR) review
We are experienced in reviewing study CSRs and ensuring accurate and thorough documentation of study findings.
Document handling
Lightning-speed document handling for timely trial initiation
OCRC streamlines every aspect of trial setup so you can launch your clinical trials in a timely and efficient manner. We achieve this through:
Rapid document preparation and submission
Our regulatory team can prepare and submit documents within 24 hours.
Quick IRB approvals
OCRC works with central Institutional Review Boards (IRBs), enabling approval within 7 to 10 days.
Efficient contract and budget turnaround
Our contract and budget team delivers a swift turnaround, preparing essential documents within 24 to 48 hours.
Contract and budget negotiations run parallel to regulatory activities, ensuring no time is lost.
Expertise in study platforms
Our site team has extensive experience with study platforms such as SIP, Florence, Impala, Inform, Imedidata Rave, Firecrest, and Greenphire, allowing immediate site registration and use upon access.
Start-up activities
Streamlined start-up activities for faster trial activation
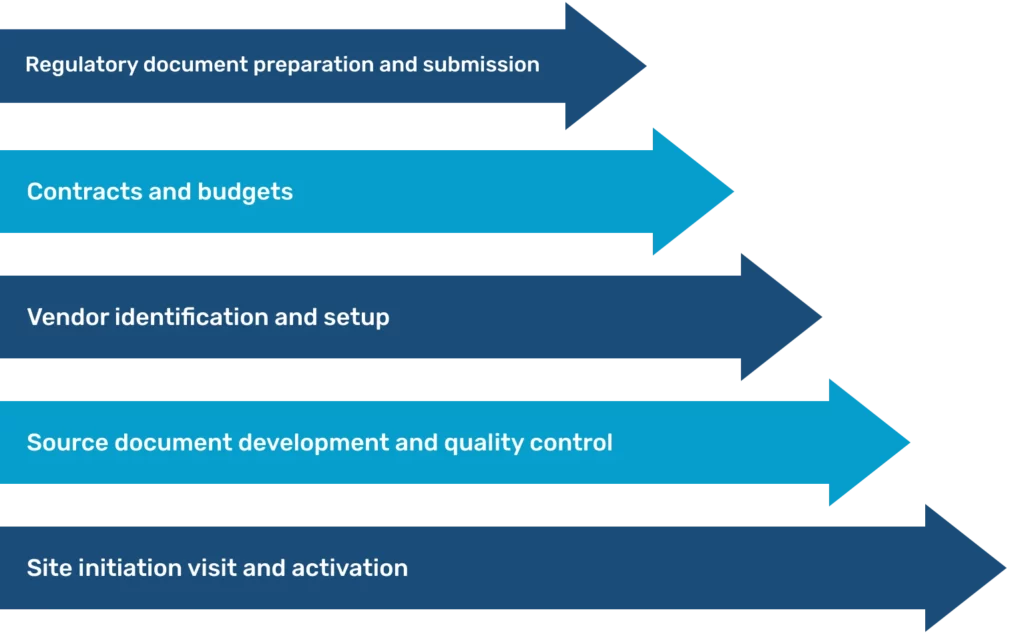
Why us for recruitment and phase 1
Recruitment and enrollment expertise
Multilingual, experienced recruiting team
Our dedicated, multilingual recruiting team brings years of experience in prescreening and enrolling patients for Phase I–IV clinical trials.
Comprehensive recruitment tools
We leverage a variety of recruitment channels, including a highly curated patient database, social media platforms (Facebook, Clinical Connection, Instagram), targeted advertising (newspapers, radio), and flyer distribution, to reach diverse patient populations.
Strong physician relationships
OCRC has cultivated strong relationships with local physicians within a 10-mile radius, who often refer eligible subjects to our facility, ensuring a steady stream of potential candidates.
Diverse and well-characterized patient base
We maintain a healthy volunteer patient base of approximately 7,500 normal volunteers. Our patient database is meticulously characterized by demographics, enabling us to quickly identify top candidates through protocol-specific queries.
Commitment to patient retention and reliability
We invest significant effort in assessing patient reliability and ensuring retention throughout studies, building trust with participants to guarantee high-quality data.
Efficient enrollment practices
As part of our standard procedure, we admit alternate candidates for each cohort to ensure that all subjects are randomized on time and studies stay on schedule.
Patient retention
Ensuring patient retention and study compliance
- Well-catered meals to accommodate diverse dietary needs
- Convenient transportation to and from the facility
- Entertainment and outdoor exercise options, as permitted by the protocol
- Comfortable living facilities to maximize comfort and relaxation
- Accommodating and friendly staff who create a welcoming atmosphere
- Essential services such as laundry and toiletries

Pharmacy
Advanced pharmacy capabilities and equipment for comprehensive trial support
Capabilities
- Compounding
- Preparation of IV infusions and subcutaneous injections
- Intranasal administration
- Topical preparation and administration
- IP packaging and labeling
- IP storage
- IP disposal
Equipment
- Laminar flow hood for sterile preparations
- Multiple refrigerators and freezers for IP storage
- −80°C freezer for IP storage
- Temperature-controlled environment
- Continuous temperature monitoring tools
Laboratory
World-class laboratory services
Efficient sample processing
We can process high-volume blood and urine samples, ensuring timely analysis and results.
Specialized handling
Our team is skilled in handling specialized samples, including those requiring flash freezing or specific processing techniques.
Reliable sample storage
Multiple −80°C freezers ensure the long-term stability of your valuable samples.
Robust infrastructure
Our laboratory is equipped with multiple centrifuges (both ambient and refrigerated), a generator backup system, and a daily supply of dry ice to maintain optimal sample integrity.
Quality assurance
We maintain meticulous records of sample harvest and quality control procedures to ensure data accuracy and regulatory compliance.
Services by external facilities
Access to specialized external services
Available procedures include:
- MRI
- CT
- DEXA
- Chest X-ray
- CPET testing
- Liver biopsies by interventional radiologists
- Optometry exams
- Retinal photography
Monitoring facilities
Superior monitoring facilities for clinical research associates
- Private monitoring rooms – accommodating up to three CRAs for focused work
- Separate unblinded monitoring area – ensuring data integrity and compliance
- Easy access to key teams – conveniently located near CRC PIs, regulatory, and data entry teams
- Essential amenities – access to copiers, high-speed internet, and scanning machines
- Remote monitoring capabilities – enabling flexible and efficient oversight
- Strategic location – proximity to John Wayne Airport for easy travel
